Abstract
Systemic sclerosis (SSc) is a refractory autoimmune disease characterized by diffuse fibrosis and vasculopathy affecting the skin, joints, and internal organs. Most immunosuppressive drugs have failed to show long-term benefit in randomized trials. In recent years, autologous hematopoietic stem cell transplantation (ASCT) has been performed to treat multiple sclerosis and SSc, however, the reconstitutions of immune components post-ASCT in SSc have not been fully described.
Subjects and Methods: We tested a total of 13 samples from 4 consenting SSc patients (3 females, 1 male) receiving IRB-approved immunoablation (TBI 600cGy) with lung shielding and Cytoxan (n=3) or Thiotepa (n=1) +/- Alemtuzumab followed by autologous CD34+ selected, cryopreserved peripheral blood hematopoietic stem cells transplantation. Their median age was 18.5-years-old (range 15.2- to 21.4-years-old). Peripheral blood samples were collected at baseline pre-ASCT, and 3, 6, 12, 24-months post-ASCT. By flow cytometry, we examined lymphocyte and monocyte subset recovery with additional focus on the FOXP3+Treg and T-helper subset reconstitution.
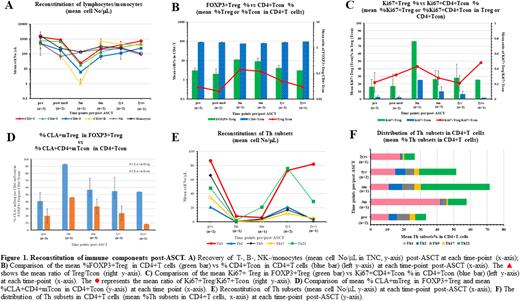
Results and Discussion: All 4 subjects experienced significant improvement in several clinical and quality of life parameters, especially in their skin disease evident within weeks after ASCT. T-cell lymphopenia was profound at 3-months; however, the recovery was evident by 6-months reaching pre-ASCT levels by 2-years post-ASCT (Fig 1A). Natural killer (NK) lymphocytes and monocytes attained the normal values by 3-months and after (Fig 1A). The FOXP3+Tregs that appeared post-ASCT displayed distinct features. There was an increased percentage of FOXP3+Tregs in total CD4+T cells during the early stage (3- to 6-months) post-ASCT (Fig 1B), leading to an increase in the ratio of Treg/Tcon (Fig 1B). The higher proportions of proliferating Tregs as measured by Ki67+FOXP3+CD4+/CD25+/CD127- T cells (Fig 1C) likely led to their increased numbers. This trend was sustained until the last evaluation at 2-years post-ASCT (Fig 1C). In contrast, CD4+Tcons showed a relatively lower proliferation rate, leading to a higher ratio of Ki67+Treg/Ki67+Tcon compared to pre-ASCT values (Fig 1C). Notably, there was an increased proportion of Tregs expressing the skin homing marker cutaneous lymphocyte-associated antigen (CLA) at 3-months lasting until the last test at 2-years post-ASCT (Fig 1D), whereas CLA+/CD4+Tcons were less plentiful.
The reconstitutions of T-helper (Th) subsets were also examined. Due to lymphopenia at the early stage post-ASCT, Th subsets were identified according to their chemokine receptor expression (Mahnke et alCytometry 2013;83(5):439-440). Gradually decreased numbers of Th2, Th9, and Th17 were observed in the peripheral pool (Fig 1E) contributing to a decline of their proportions in total CD4+T (Fig 1F). Nevertheless, absolute number of Th1 and Th22 cells returned to levels seen pre-ASCT without any recurrence or flare of SSc symptoms. The CD45RO+ memory phenotype was dominant during the early stages post-ASCT in all subsets (data not shown).
Summary: Successful reconstitution of lymphocytes and monocytes post-ASCT was observed in available SSc patients. Post-ASCT circulating FOXP3+Tregs displayed distinct features where the increased number led to an elevated Treg/Tcon ratio in the peripheral pool. Amongst Tregs, the skin homing CLA+ subset was further enriched correlating with improved skin scores in all patients. Improved peripheral proliferation and increased expression of CLA+ Tregs may provide protection from skin disease recurrence despite parallel Tcon reconstitution. The decrease of the Th2/Th9/Th17 subsets in the peripheral pool post-ASCT possibly contributes to the clinically less autoimmune phenotype. These results need to be validated in future patients.
Acknowledgements: We thank all participating patients, nurses, physicians, and scientists in this study for their contributions. The study was supported by the "Idea Development Award" from the Department of Defense - Scleroderma Research Program (Award number W81XWH-21-1-0782 and W81XWH-21-1-0783).
Disclosures
Szabolcs:Forge Biologics, Inc: Patents & Royalties.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal